Secondary raw materials / Lithium-ion battery recycling
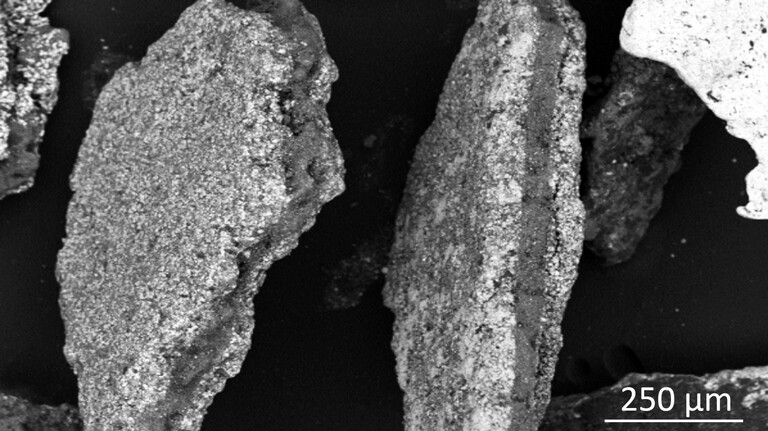
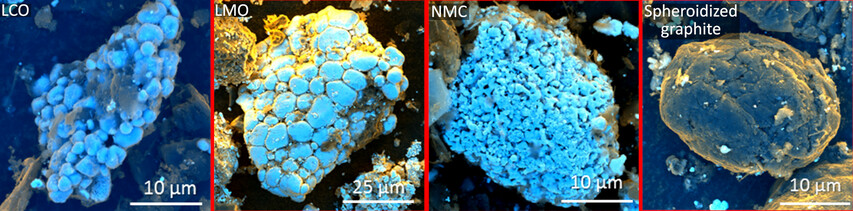
Automated material characterization is one of the novel key characterization methods to understand recycling processes. This is because – compared to simple chemical assays (e.g. XRF, ICP-OES…) or quantitative X-ray diffraction – MLA or QEMSCAN analyses provide additional information relevant for processing. This includes information on modal phase composition, liberation, phase locking and association, elemental deportment, as well as physical material properties such as the particles shape, size and densities.
Due to their high energy density, lithium-ion batteries (LIB) rapidly became the dominant electrochemical power source for portable electronics since their commercialization in the 1990’s. Many devices, including consumer electronics such as mobile phones and laptops, hybrid and electric vehicles, and stationary storage applications, use these batteries. Considering that LIBs have been in use for 30 years, and as their production volume continues to increase, it is reasonable to forecast a considerable growth of end-of-life LIB streams in the foreseeable future.
The recycling of LIBs, e.g., the joint recovery of several valuable components, remains challenging mainly because of their complex composition and structure. For example, the presence of binder complicates the liberation of active particles from the foils, reducing the liberation efficiency during the recycling process. An extended mechanical process may be required to liberate more active particles but could result as well in undesired foil particles in the fine fraction. The diversity of cathode chemistry poses yet another challenge since different battery chemistries may require specific conditions for an efficient recycling process. Impurities such as foils as well as different cathode chemistries can be problematic in subsequent recycling steps, such as hydrometallurgical processes. Therefore, knowing the exact modal composition and elemental deportment of valuable components as well as crucial parameters such as the liberation of foils, cathode active materials and graphite - in short, an accurate characterization of spent LIBs - is essential for monitoring and improving the efficiency of recycling processes.
References
Vanderbruggen, A., Gugala, E., Blannin, R., Bachmann, K., Serna-Guerrero, R., Rudolph, M., 2021. Automated mineralogy as a novel approach for the compositional and textural characterization of spent lithium-ion batteries. Miner. Eng. 169, 106924. doi.org/10.1016/j.mineng.2021.106924
Vanderbruggen, A., Sygusch, J., Rudolph, M., Serna-Guerrero, R., 2021. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surfaces A Physicochem. Eng. Asp. 626, 127111. doi.org/10.1016/j.colsurfa.2021.127111